
We can therefore confirm their order of reactivity (from most reactive to least) as: potassium, magnesium then zinc. We’d expect the potassium to react explosively (in reality you would never do this in a school laboratory as it is too dangerous), while the magnesium would bubble vigorously and the zinc would form bubbles very slowly. Let’s say we carry out the experiment described above by adding potassium, magnesium and zinc into test tubes containing dilute sulfuric acid. Chemical Changes - GCSE Chemistry Paper 1 Worksheet Bundle.
#REACTIVITY SERIES CHEMISTRY GCSE SERIES#
Review these Gen-Chem topics for Organic Chemistry with Video Series + Quiz. A simple form of it is shown here: sodium. The faster the bubbles are given off, the faster the rate of reaction and the more reactive the metal. 2: Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules. The reactivity series shows the list of metals starting with the most reactive at the top and the least reactive at the bottom. The bubbles are hydrogen gas and can be confirmed using a lit splint, which will produce a ‘squeaky pop’ when the hydrogen burns. It is important that each metal has the same surface area because this will affect the rate of reaction.Ĭount the number of bubbles produced in a given time. Sulfate ions, SO 4 2-, appear on both sides of the equation because they do not take part in the reaction.Add equal volumes of dilute hydrochloric acid or dilute sulfuric acid into a series of test tubes then add a equal mass of metal to each test tube. It is a watered-down version of the Electrochemical Series. The chemical properties of a metal and its compounds. This practice sheet covers part of Topic 4 of Chemistry Chemical Changes Its designed for both GCSE Triple Award Science (separate sciences) and Double. The reactivity series of metals shows how reactive some elements are compared with others. Understanding the reactivity series is fundamental to chemistry. Energy changes in chemistry - (CCEA) Gas chemistry - (CCEA). The reactivity series lists elements (mostly metals) in order of decreasing reactivity. Mg(s) + Cu 2+ (aq) + SO 4 2- (aq) → Mg 2+ (aq) + SO 4 2- (aq) + Cu(s) The Reactivity Series is a list of metal elements in order of how easily they can lose electrons to form positive ions. When metals are arranged in order of decreasing chemical reactivity, the reactivity series is obtained. The reactivity series of metals is a chart listing metals in order of decreasing reactivity.

The equation below shows the ions present on both sides of the equation: It is used to determine the products of single.
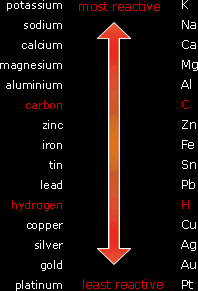
Here is the balanced ionic equation for the reaction between magnesium and copper(II) sulfate. In chemistry, the reactivity series is a series of metals, in order of reactivity from highest to lowest. Magnesium is the most reactive metal from these three as it undergoes two reactions, iron is next and copper is the least reactive.ĭisplacement reactions as redox reactions The table below shows the results of reacting different metals with different salt solutions. GCSE Science Revision Chemistry 'The Reactivity Series' Freesciencelessons 683K subscribers Join 6.4K 533K views 6 years ago AQA GCSE Chemistry Paper 1 Chemical Reactions Workbooks for. You can work out a reactivity series for many substances by carrying out displacement reactions.


 0 kommentar(er)
0 kommentar(er)
